Abstract
Myeloproliferative neoplasms are associated with significant alterations in the bone marrow microenvironment that contribute to disease pathogenesis. The most striking alteration is the development of myelofibrosis, which is characterized by extensive collagen deposition in the bone marrow and is associated with a poor prognosis. Recent evidence suggests that expression of key niche factors, including CXCL12 (stromal derived factor-1, SDF-1) and Kit ligand are reduced in MPNs. This is relevant, since studies by our group and others have shown that deleting these niche factors from stromal cells results in a shift in hematopoiesis from the bone marrow to spleen. Indeed, a prominent feature of MPN is the development of splenomegaly and extramedullary hematopoiesis. There is evidence implicating inflammatory mediators in the development of myelofibrosis. In particular, increased production of TGF-β produced by megakaryocytes and monocytes is found in most patients with MPNs. To assess the role of TGF-β signaling in mesenchymal stromal cells in the bone marrow in the development of myelofibrosis, we generated Osx-Cre; Tgfbr2 f/- mice, in which TGF-β signaling is abrogated in all bone marrow mesenchymal stromal cells (including Lepr + stromal cells), but not endothelial cells or hematopoietic cells. We transplanted MPL W515L transduced hematopoietic stem and progenitor cells (HSPCs) or JAK2 V617F bone marrow into these mice and quantified myelofibrosis using reticulin staining and Collagen 1 and 3 immunostaining. We previously reported that deletion of TGF-β signaling in mesenchymal stromal cells in these mice abrogated the development of myelofibrosis, and we presented evidence that this was mediated by non-canonical JNK-dependent TGF-β signaling. Here, we describe the impact of stromal TGF-β signaling on the bone marrow hematopoietic niche in MPN.
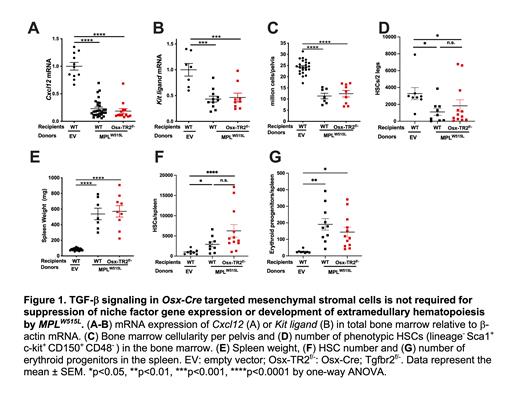
MPL W515L transduced HSPCs were transplanted into Osx-Cre; Tgfbr2 f/- mice, and the impact on hematopoietic niche disruption and development of extramedullary hematopoiesis was assessed. In control recipients, transplantation of MPL W515L HSPCs resulted in marked decreases in bone marrow Cxcl12 and Kit ligand expression (Figure 1A-B). Surprisingly, a similar decrease was observed in Osx-Cre; Tgfbr2 f/- recipients. The loss of these key niche factors is predicted to impair hematopoietic niche function in the bone marrow. Consistent with this prediction, total bone marrow cellularity and HSC number were significantly reduced in both control and Osx-Cre; Tgfbr2 f/- recipients (Figure 1C-D). Finally, disruption of the bone marrow niche is often associated with extramedullary hematopoiesis. Indeed, a significant increase in spleen size and spleen HSCs and erythroid progenitors was observed in control recipients (Figure 1E-G). Again, a similar phenotype was observed in Osx-Cre; Tgfbr2 f/- recipients.
Collectively, these data show that TGF-β signaling in bone marrow mesenchymal stromal cells is required for the development of myelofibrosis but not hematopoietic niche disruption in MPNs. Thus, these data show for the first time that the signals that induce a fibrogenic program in bone marrow mesenchymal stromal cells are distinct from those that suppress Cxcl12 and Kit ligand expression. Our data show that the fibrogenic program is dependent on non-canonical JNK-dependent TGF-β signaling, while the signals that regulate niche factor expression remain unknown.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal